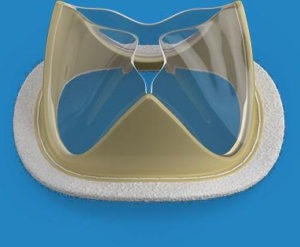
Foldax, Inc. announced its first-in-human use of the Tria heart valve under its FDA Early Feasibility Study (EFS) for the treatment of aortic valve disease. Clinicians at Beaumont Hospital, Royal Oak, in Michigan, implanted this innovative flexible polymer heart valve, which has the potential to address durability and thrombogenicity issues of currently available heart valves.
Dr. Marc Sakwa, Beaumont’s Chief of Cardiovascular Surgery, said, “The procedure was successfully performed on July 30th and the patient is doing well and has been discharged.”
“The start of our EFS study in the US represents a major milestone for Foldax and heart valve patients worldwide since Tria valves represent true next generation technology. The Aortic EFS combined with our progress toward mitral and transcatheter versions of the valves next year have the potential to revolutionize the heart valve industry.” – Ken Charhut, Foldax Executive Chairman
Tria heart valves combine LifePolymer™, an advanced biopolymer material, and a patented design to create a valve with the potential for lowering the cost of medical care given the increasing costs of using animal tissue valves and their associated durability and calcification concerns. The proprietary biopolymer material and design of the Tria heart valves also allows for high volume manufacturing. The valves are robotically manufactured to provide the highest level of quality and precision and allow for future patient customization, while eliminating the variability of human production.
“The start of our EFS study in the US represents a major milestone for Foldax and heart valve patients worldwide since Tria valves represent true next generation technology. The Aortic EFS combined with our progress toward mitral and transcatheter versions of the valves next year have the potential to revolutionize the heart valve industry. We are bringing 21st Century solutions to the worldwide problem of providing high quality products at an affordable price,” stated Ken Charhut, Foldax Executive Chairman.
Foldax’s Tria valves were developed by drawing on expertise from the Commonwealth Scientific and Industrial Research Organization (CSIRO) for proprietary polymer development and Caltech’s Division of Engineering and Applied Science and Chemistry Department. The Company’s investors include Kairos Ventures and Biostar Capital.
The complete Tria heart valve platform will include valves developed for use in aortic and mitral valve disease with transcatheter and surgical applications. The company plans to complete enrollment in the Aortic EFS study at Beaumont Hospital and two additional sites this year.